The role of conserved arginine in the GH70 family: a computational study of the structural features and their implications on the catalytic mechanism of GTF-SI from Streptoccocus mutans†
Abstract
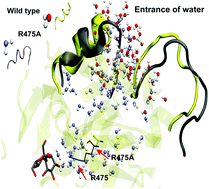
In this work, molecular dynamics and QM/MM calculations were employed to examine the structural and catalytic features of the retaining glucosyltransferase GTF-SI from the GH70 family, which participates in the process of caries formation. Our goal was to obtain a deeper understanding of the role of R475 in the mechanism of sucrose breakage. This residue is highly conserved in the GH70 family and so far there has been no evidence that shows what could be the role of this residue in the catalysis performed by GTF-SI. In order to understand the structural role of R475 in the native enzyme, we built full enzyme models of the wild type and the mutants R475A and R475Q. These models were addressed by means of molecular dynamics simulations, which allowed the assessment of the dynamical effect of the R475 mutation on the active site. Then, representative structures were chosen for each one of the mutant models and QM/MM calculations were carried out to unravel the catalytic role of R475. Our results show that the R475 mutation increases the flexibility of the enzyme, which triggers the entrance of water molecules in the active site. In addition, QM/MM calculations indicate that R475 is able to provide a great stabilization to the carboxylate moiety of the acid/base E515, which is an essential characteristic favoring the proton transfer process that promotes the glycosidic bond breakage of sucrose.


 Please wait while we load your content...
Please wait while we load your content...