Tautomerism and stereodynamics in Schiff bases from gossypol and hemigossypol with N-aminoheterocycles†‡
Abstract
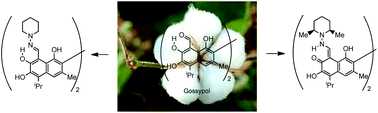
Tautomerism plays a pivotal role in structural stabilization and reactivity. Herein we investigate in detail, aided by DFT simulations, the case of gossypol, a naturally occurring atropisomeric dialdehyde showing promising properties as a male contraceptive and an antineoplasic agent. Its toxicity linked to reactive aldehydo groups can be reduced through amino conjugation. The occurrence of either imino or enamino structures is puzzling indeed and a clear-cut rationale is missing yet. N-enamine–N-enamine structures are prevalent or exclusive tautomers for Schiff bases from gossypol, while their corresponding hydrazones only possess N-imine–N-imine structures both in solution and the solid state. The modification of interactions between the lone pairs on the nitrogen atoms by altering the steric hindrance of the non-iminic nitrogen can favor enamine tautomers. This assumption has now been confirmed and, in the solid state, hydrazones from N-aminopiperidine and their cis-2,6-dimethylderivative present bis-imine and bis-enamine structures, respectively. In solution, these compounds exist in equilibrium between both structures. The tautomerization mechanism, analysis of axial chirality and aromaticity in such H-bonded pseudorings are discussed as well.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...