Diversity of mechanisms in Ras–GAP catalysis of guanosine triphosphate hydrolysis revealed by molecular modeling†
Abstract
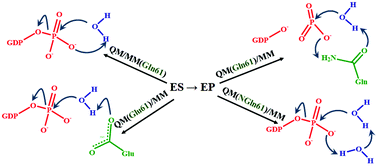
The mechanism of the deceptively simple reaction of guanosine triphosphate (GTP) hydrolysis catalyzed by the cellular protein Ras in complex with the activating protein GAP is an important issue because of the significance of this reaction in cancer research. We show that molecular modeling of GTP hydrolysis in the Ras–GAP active site reveals a diversity of mechanisms of the intrinsic chemical reaction depending on molecular groups at position 61 in Ras occupied by glutamine in the wild-type enzyme. First, a comparison of reaction energy profiles computed at the quantum mechanics/molecular mechanics (QM/MM) level shows that an assignment of the Gln61 side chain in the wild-type Ras either to QM or to MM parts leads to different scenarios corresponding to the glutamine-assisted or the substrate-assisted mechanisms. Second, replacement of Gln61 by the nitro-analog of glutamine (NGln) or by Glu, applied in experimental studies, results in two more scenarios featuring the so-called two-water and the concerted-type mechanisms. The glutamine-assisted mechanism in the wild-type Ras–GAP, in which the conserved Gln61 plays a decisive role, switching between the amide and imide tautomer forms, is consistent with the known experimental results of structural, kinetic and spectroscopy studies. The results emphasize the role of the Ras residue Gln61 in Ras–GAP catalysis and explain the retained catalytic activity of the Ras–GAP complex towards GTP hydrolysis in the Gln61NGln and Gln61Glu mutants of Ras.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...