1,2,4-Oxadiazole-5-ones as analogues of tamoxifen: synthesis and biological evaluation†
Abstract
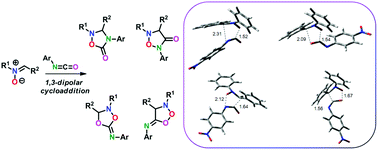
A series of 2,3,4-triaryl-substituted 1,2,4-oxadiazole-5-ones have been prepared as fixed-ring analogues of tamoxifen (TAM), a drug inhibitor of Estradiol Receptor (ER) used in breast cancer therapy, by an efficient synthetic protocol based on a 1,3-dipolar cycloaddition of nitrones to isocyanates. Some of the newly synthesized compounds (14d–f, 14h and 14k) show a significant cytotoxic effect in a human breast cancer cell line (MCF-7) possessing IC50 values between 15.63 and 31.82 μM. In addition, compounds 14d–f, 14h and 14k are able to increase the p53 expression levels, activating also the apoptotic pathway. Molecular modeling studies of novel compounds performed on the crystal structure of ER reveal the presence of strong hydrophobic interactions with the aromatic rings of the ligands similar to TAM. These data suggest that 1,2,4-oxadiazole-5-ones can be considered analogues of TAM, and that their anticancer activity might be partially due to ER inhibition.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...