Oxidative sulfonamidomethylation of imidazopyridines utilizing methanol as the main C1 source†
Abstract
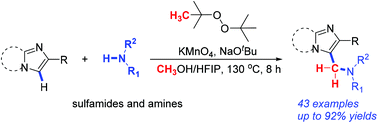
An efficient one pot, three component synthesis of C3 sulfonamidomethylated imidazopyridines has been disclosed under metal-free conditions, which utilized the commercially available and renewable reagent methanol as the main methylene source. A wide range of substituted imidazopyridines and sulfamides/amines were well tolerated to afford the corresponding products in up to 92% yield. In the isotopic labelling experiment, it was found that a minor part of the methylene also originated from DTBP. Moreover, the radical scavenger reactions were conducted, which suggested that a free-radical mechanism was probably not involved. The current methodology featured several advantages, including broad substrate scope, good functional group tolerance and high reaction efficiency.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...