Solution-phase total synthesis of teixobactin†
Abstract
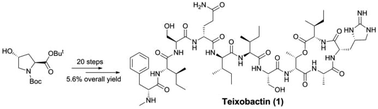
The first solution-phase total synthesis of the cyclic depsipeptide teixobactin is described. Stereoselective construction of L-allo-enduracididine was established, and the protective groups for the peptide coupling reactions and conditions for the assembly of the fragments were also optimised. The longest linear sequence for the total synthesis was 20 steps from the known L-cis-4-hydroxyproline derivative and gave a 5.6% overall yield. This solution-phase total synthesis could serve as a complement to the current solid-phase synthesis of teixobactin.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...