Iron-catalyzed asymmetric intramolecular cyclopropanation reactions using chiral tetramethyl-1,1′-spirobiindane-based bisoxazoline (TMSI-BOX) ligands†
Abstract
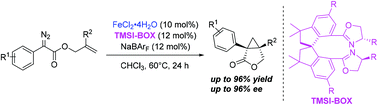
The versatile application of chiral bisoxazoline (BOX) ligands in diverse metal-catalyzed asymmetric reactions results in growing demand for novel BOX ligands containing different motifs. Herein, the successful development of a chiral spiro bisoxazoline ligand (TMSI-BOX) on the basis of the tetramethyl-1,1′-spirobiindane motif and bisoxazoline chelating units is described. The corresponding Fe complexes of TMSI-BOX proved to be excellent catalysts in the asymmetric intramolecular cyclopropanation reaction of diazo derivatives, providing synthetically versatile [3.1.0]bicycloalkane derivatives bearing two contiguous quaternary chiral centers with high enantiomeric purity.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...