Design, synthesis and glycosidase inhibition studies of novel triazole fused iminocyclitol-δ-lactams†
Abstract
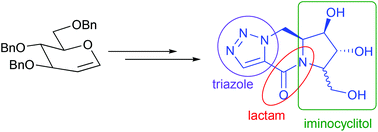
Synthesis of novel triazole fused iminocyclitol-δ-lactams is described. The synthetic sequence involves the intermolecular [3 + 2] cycloaddition reaction of five-membered iminocyclitol derived azides with diethylacetylene dicarboxylate followed by intramolecular lactamisation, decarboxylation/reduction and final deprotection. Compound 3 is found to be a selective inhibitor of α-glucosidase from baker's yeast while two other compounds (2 and 4) that possess an additional hydroxymethyl group in the triazole ring are selective against β-galactosidase from E. coli. Docking studies suggest the significance of the lactam carbonyl group for effective binding of these inhibitors with the active sites through hydrogen bonding.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...