Cascade Knoevenagel and aza-Wittig reactions for the synthesis of substituted quinolines and quinolin-4-ols†‡
Abstract
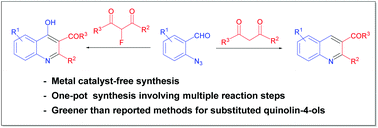
A [4 + 2] annulation involving cascade Knoevenagel, aza-Wittig and dehydrofluorination reactions is developed for the synthesis of substituted quinolin-4-ols including analogs bearing CF2H, CF3, and C2F5 groups. This simple and highly efficient method is also applicable for the synthesis of substituted quinolines. A number of reported biologically active compounds can be readily prepared by this one-pot synthesis. Green chemistry metrics analysis of the new reaction processes provided favorable results.


 Please wait while we load your content...
Please wait while we load your content...