An innovative synthesis pathway to benzodioxanes: the peculiar reactivity of glycerol carbonate and catechol†
Abstract
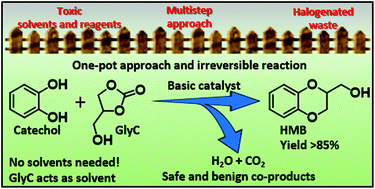
A peculiar reactivity of glycerol carbonate (GlyC) as an innovative and highly reactive alkylating agent for phenolic compounds is investigated in this article. In particular, 2-hydroxymethyl-1,4-benzodioxane (HMB), a key intermediate for the pharmaceutical industry, has been selectively synthesized by the reaction of a slight excess of GlyC with catechol in the presence of a basic catalyst (NaOCH3, Na-mordenite, MgO), without requiring a reaction solvent. Both reagents have been quantitatively converted in just one hour at 170 °C with a HMB yield, up to 88%, in the presence of a homogeneous basic catalyst (NaOCH3). Notably, the main side product, the HMB isomer, may be an interesting intermediate for the synthesis of calone analogues, which are important scaffolds used in fragrances. A detailed mechanistic study, supported by kinetics, GC-MS, and HMBC NMR characterization, is also reported. Accordingly, this paper describes a completely innovative and greener synthesis pathway to benzodioxanes.


 Please wait while we load your content...
Please wait while we load your content...