Deep eutectic solvents as extraction media for metal salts and oxides exemplarily shown for phosphates from incinerated sewage sludge ash†
Abstract
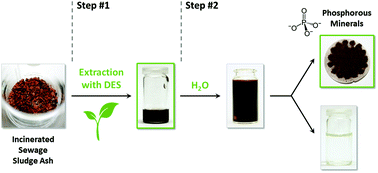
Deep eutectic solvents (DESs) based on natural products can be used as selective extraction media for a wide variety of metal salts and oxides and specifically for phosphates from incinerated sewage sludge ash (ISSA). The solubility behavior of the main components of ISSA as well as several metal salts and metal oxides in DESs is investigated and an empirical basis for possible applications is provided. The solubility can partly be correlated with the approximated pH value of the DESs: the tested phosphates and metal chlorides dissolve preferably in DESs containing urea or N,N′-dimethyl urea (DMU), which offer neutral to less basic conditions; in general, metal oxides enrich in DESs with carboxylic acids at a highly acidic pH value; and acetates dissolve best in DESs based on choline chloride (ChCl). From these findings, DESs were tested as environmentally friendly extraction media for phosphorus from ISSA and the first results show the feasibility of the approach, which may lead to a low-energy process. With the DES DMU/mannose (5 : 1), an accumulation of phosphorus up to 31% was achieved, emphasizing the potential of biodegradable DESs as extraction media.


 Please wait while we load your content...
Please wait while we load your content...