A base promoted one pot solvent free version of the Ramachary reductive coupling/alkylation reaction for the synthesis of 2,2-disubstituted ethyl cyanoacetates†
Abstract
An N,N-diisopropylethylamine promoted solvent-free Ramachary reductive coupling/alkylation (RRC/A) reaction for the synthesis of 2,2-disubstituted ethyl cyanoacetates has been developed. A series of 2,2-disubstituted ethyl cyanoacetates were synthesized in one pot by the RRC/A reaction of commercially available aldehydes, ethyl cyanacetates, alkyl halides and Hantzsch ester. A solvent free two step multicomponent reaction has also been developed for the preparation of 2,2-dialkylated malononitriles and 2,2-dialkylated 4-nitrophenyl acetonitriles. All the designed RRC/A products could be easily obtained with good yields by these methods.
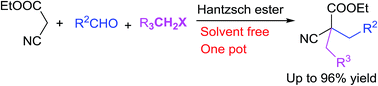


 Please wait while we load your content...
Please wait while we load your content...