Synergetic effects of bimetals in modified beta zeolite for lactic acid synthesis from biomass-derived carbohydrates†
Abstract
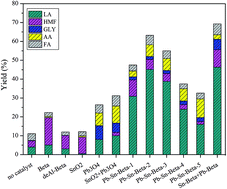
An experimental study was carried out to convert carbohydrates using bimetal modified beta zeolite to obtain a maximum yield of lactic acid. The relationship between the properties and the catalytic performance of various bimetal modified beta zeolites was evaluated. The results showed that the maximum yield of lactic acid reached 52% with more than 99% glucose conversion over Pb–Sn-beta (0.3 mmol g−1, Pb/Sn = 4 : 7) at 190 °C for 2 h under ambient air pressure. To evaluate the synergetic mechanism of lead and tin, key intermediates such as fructose, dihydroxyacetone, glyceraldehyde and pyruvaldehyde were used as probe reactants that were catalyzed by Pb-beta, Sn-beta and Pb–Sn-beta. The revealed key role of lead was to promote the isomerization of glucose to fructose and the retro-aldol condensation reaction from fructose to dihydroxyacetone and glyceraldehyde; meanwhile, tin had a superior catalytic performance in the dehydration of dihydroxyacetone, the hydration of pyruvaldehyde and the isomerization of pyruvic aldehyde hydrate.


 Please wait while we load your content...
Please wait while we load your content...