Transition-metal-free cleavage of C–C double bonds: a three-component reaction of aromatic alkenes with S8 and amides towards aryl thioamides†‡
Abstract
A convenient and novel transition-metal-free cleavage of unstrained C–C double bonds has been developed from a three-component reaction involving aromatic alkenes, S8 and amides, which selectively provides a variety of aryl thioamides in up to 96% yields. This strategy features a simple operation and wide substrate scope. Note that internal aromatic alkenes and/or inactive acetamides could be applicable to this protocol.
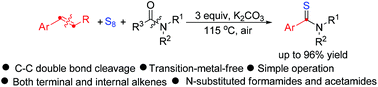


 Please wait while we load your content...
Please wait while we load your content...