Pd/Gorlos-Phos-catalyzed cross-coupling between two different aryl chlorides in the presence of B2Pin2 and cytotoxicity studies of the products†
Abstract
With the readily available Gorlos-Phos as a ligand, unsymmetrical biaryls were prepared efficiently from Pd-catalyzed one-pot two-step cross-coupling of two different aryl chlorides in the presence of B2Pin2, tolerating functional groups such as aldehyde, ketone, ester, ether, nitrile, amide, fluorine, sulphonyl and trifluoromethyl groups. Besides the substituted phenyl chlorides, hetero-aryl chlorides, 1-chloronaphthalene, and dichlorobenzenes could also be applied. The cytotoxicity against human lung cancer A549 cells for some of the compounds has been studied.
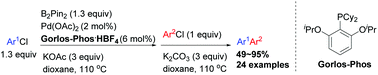


 Please wait while we load your content...
Please wait while we load your content...