A facile approach to synthesize azaindoline functionalized spirocarbocyclic scaffolds via a Pd-catalyzed cascade cyclization/dearomatization process†
Abstract
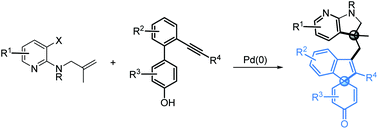
A novel construction of azaindoline functionalized spirocarbocyclic scaffolds bearing two all-carbon quaternary centers has been developed via a palladium-catalyzed Heck cyclization/phenol dearomatization reaction. In the cascade process, three new C(sp2)–C(sp3) bonds are formed in a single chemical operation. The approach shows broad substrate scope and good functional-group tolerance.


 Please wait while we load your content...
Please wait while we load your content...