Preparation and utilization of a sub-5 nm PbO2 colloid as an excellent co-catalyst for Pt-based catalysts toward ethanol electro-oxidation
Abstract
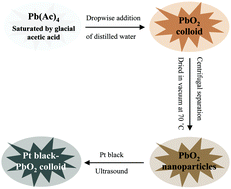
In order to improve the utilization and performance of Pt-based catalysts used in direct ethanol fuel cells, a commercial Pt black catalyst was modified by PbO2 nanoparticles with a mean size of 4.59 nm. Electrocatalytic tests demonstrate that the as-prepared Pt black–PbO2 hybrid catalyst possesses a much higher catalytic activity and longer durability than commercial Pt black for ethanol electro-oxidation. Specifically, the Pt black–PbO2 hybrid showed an onset potential that was about 98 mV less positive, together with a peak current density 4 times higher than that observed for the commercial Pt black catalyst in the cyclic voltammetry tests. The ratio of current densities per unit Pt mass on the Pt black–PbO2 and Pt black catalysts is 35.5 : 1 for the long-term (2 hours) chronoamperometric experiments measured at −0.4 V (vs. SCE). The in situ FTIR spectroscopic studies reveal that the Pt black–PbO2 (4 : 1) catalyst exhibits excellent activity for breaking the C–C bond of ethanol, which is 3.21 times higher than that of the Pt black catalyst at 0 V (vs. SCE).


 Please wait while we load your content...
Please wait while we load your content...