Pyrene–antipyrine based highly selective and sensitive turn-on fluorescent sensor for Th(iv)†
Abstract
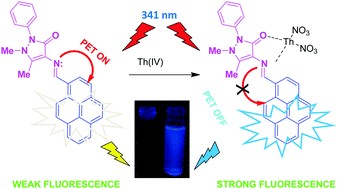
Herein, a turn-on fluorescent sensor for thorium(IV) based on pyrene–antipyrine unit is described. The chemosensor 1,5-dimethyl-2-phenyl-4-[(pyren-1-ylmethylene)amino]-1H-pyrazol-3-one (PYAN) exhibited weak fluorescence due to quenching by photoinduced electron transfer (PET) from the lone pair of electrons on the nitrogen atom of imine group in pyrene. The probe PYAN shows an emission maximum at 446 nm, and exhibits fluorescence enhancement with blue emission exclusively in the presence of Th(IV) in CH3CN : H2O (50 : 50, v/v) solution. Besides, the gradual addition of Th(IV) to PYAN induces a visual color change from yellow to colorless with concomitant blue shift from 446 to 418 nm in the fluorescence spectra. It is proposed that the blockage of PET by Th(IV) ion complexation induced emission enhancement. Recognition mechanism based on PET was confirmed through lifetime studies, 1H NMR titration and theoretical calculations. It was observed that 1 : 1 stoichiometry complex is formed between Th(IV) and PYAN with the binding constant of 4.5 × 104 L mol−1. The limit of detection was found to be as low as 4.9 nM. The probe could also be conveniently applied for the detection of Th(IV) in real samples.


 Please wait while we load your content...
Please wait while we load your content...