Gas phase hydration of halogenated benzene cations. Is it hydrogen or halogen bonding?†
Abstract
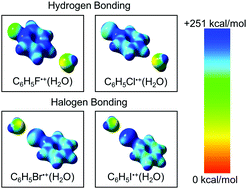
Halogen bonding (XB) non-covalent interactions can be observed in compounds containing chlorine, bromine, or iodine which can form directed close contacts of the type R1–X⋯Y–R2, where the halogen X acts as a Lewis acid and Y can be any electron donor moiety including electron lone pairs on hetero atoms such as O and N, or π electrons in olefin double bonds and aromatic conjugated systems. In this work, we present the first evidence for the formation of ionic halogen bonds (IXBs) in the hydration of bromobenzene and iodobenzene radical cations in the gas phase. We present a combined thermochemical investigation using the mass-selected ion mobility (MSIM) technique and density functional theory (DFT) calculations of the stepwise hydration of the fluoro, chloro, bromo, and iodobenzene radical cations. The binding energy associated with the formation of an IXB in the hydration of the iodobenzene cation (11.2 kcal mol−1) is about 20% higher than the typical unconventional ionic hydrogen bond (IHB) of the CHδ+⋯OH2 interaction. The formation of an IXB in the hydration of the iodobenzene cation involves a significant entropy loss (29 cal mol−1 K−1) resulting from the formation of a more ordered structure and a highly directional interaction between the oxygen lone pair of electrons of water and the electropositive region around the iodine atom of the iodobenzene cation. In comparison, the hydration of the fluorobenzene and chlorobenzene cations where IHBs are formed, −ΔS° = 18–21 cal mol−1 K−1 consistent with the formation of less ordered structures and loose interactions. The electrostatic potentials on the lowest energy structures of the hydrated halogenated benzene radical cations show clearly that the formation of an IXB is driven by a positively charged σ-hole on the external side of the halogen atom X along the C–X bond axis. The size of the σ-hole increases significantly in bromobenzene and iodobenzene radical cations which results in strong interaction potentials with the electron lone pairs of the oxygen atom of the water molecules and thus IXBs provide the most stable hydrated structures of the bromobenzene and iodobenzene radical cations. The results clearly distinguish the hydration behaviors resulting from the ionic hydrogen and halogen bonding interactions of fluorobenzene and iodobenzene cations, respectively, and establish the different bonding and structural features of the two interactions.


 Please wait while we load your content...
Please wait while we load your content...