Multi-functionalized herringbone carbon nanofiber for anodes of lithium ion batteries†
Abstract
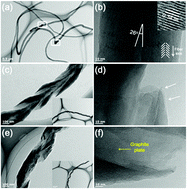
Herringbone carbon nanofibers (HCNFs) are prepared for use as anode materials in lithium-ion batteries (LIBs). HCNFs are prepared using a Ni–Fe catalyst and subsequently multi-functionalized with oxygen using the Hummers’ method, and then with both oxygen and nitrogen-containing 2-ureido-4[1H]pyrimidinone (UHP) moieties, which endow the HCNFs with the ability to form quadruple hydrogen bonds (QHBs). The as-prepared HCNFs are, on average, 13 μm in length and 100 nm in diameter, with a highly graphitic structure. The oxidized HCNFs (Ox-HCNFs) obtained by Hummers' method are partially exfoliated, having double-bladed saw-like structures that extend in the direction of the graphite planes. QHBs are formed between the HCNFs after functionalization with the UHP moieties. The final surface-modified HCNFs (N-Ox-HCNFs) have more electrochemical sites, shorter Li+ diffusion lengths, and additional electron pathways compared with the as-prepared HCNF and Ox-HCNF. The introduction of oxygen- and nitrogen-containing functional groups improves the performance of LIBs: a high charge capacity of 763 mA h g−1 at 0.1 A g−1, excellent rate capability (a capacity of 402 mA h g−1 at 3 A g−1), and near 100% capacity retention after 300 cycles are reported.


 Please wait while we load your content...
Please wait while we load your content...