Mechanism, chemoselectivity and enantioselectivity for the rhodium-catalyzed desymmetric synthesis of hydrobenzofurans: a theoretical study†
Abstract
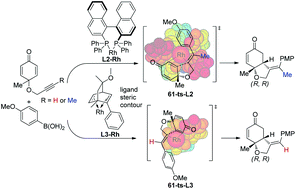
Rhodium-catalyzed desymmetrization of cyclohexadienones is an efficient method for the asymmetric synthesis of hydrobenzofurans. The newly reported density functional theory (DFT) method MN12-L is used to investigate the mechanism, chemoselectivity and enantioselectivity for this type of reaction. Computational results indicate that the preferred pathway involves transmetallation to form an aryl–rhodium compound, alkyne insertion, intramolecular olefin insertion, and protonation to generate the hydrobenzofuran product. The enantioselectivity is controlled by the intramolecular olefin insertion step, which is ascribed to the steric repulsions between the ligand and substrate. In addition, the generation of a side product via a second intermolecular alkyne insertion has also been considered in calculations.

 Please wait while we load your content...
Please wait while we load your content...