Palladium-catalyzed C(sp3)–H arylation of lactic acid: efficient synthesis of chiral β-aryl-α-hydroxy acids†
Abstract
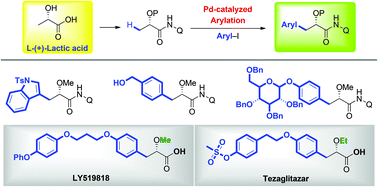
A Pd-catalyzed arylation of lactic acid employing 8-aminoquinoline as the directing group has been reported. The protocol is found to be compatible with a broad range of synthetically useful functional groups, thus providing a practical route to chiral β-aryl-α-hydroxy acids. Further, the new reaction has also been applied to the synthesis of pharmaceutically important α-hydroxy acids, such as LY519818 and tesaglitazar.
- This article is part of the themed collection: 2015 Emerging Investigators by OCF

 Please wait while we load your content...
Please wait while we load your content...