A direct and vicinal functionalization of the 1-methyl-2-quinolone framework: 4-alkoxylation and 3-chlorination†
Abstract
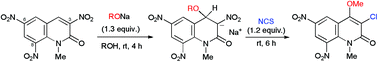
Bis(functionalization), 4-alkoxylation and 3-chlorination, of the 1-methyl-2-quinolone framework was achieved under mild conditions by a sequential treatment of 3-nitrated 1-methyl-2-quinolones with sodium alkoxide and N-chlorosuccinimide. Moreover, a succinimide group instead of an alkoxy group was introduced at the 4-position, affording a masked form of the 4-amino-3-chloro-2-quinolone derivative. Furthermore, the prepared vicinally functionalized quinolones thus obtained were subjected to a Suzuki–Miyaura coupling reaction, arylating the 3-position.


 Please wait while we load your content...
Please wait while we load your content...