New electron-donor and -acceptor architectures from benzofurazans and sym-triaminobenzenes: intermediates, products and an unusual nitro group shift†
Abstract
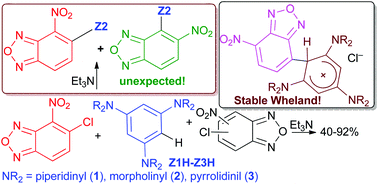
New all-conjugated C–C coupling products bearing both an electron-poor and an electron-rich aromatic moiety have been obtained from the reaction between sym-triaminobenzene derivatives and a series of isomeric chloro-nitrobenzofurazans. The reactions occur under mild reaction conditions, and in some cases a different behaviour depending on the presence, or not, of triethylamine was observed. From 1,3,5-tris(N-morpholinyl)benzene and 5-chloro-4-nitrobenzofurazan in the presence of triethylamine an unexpected product derived from the shift of the nitro group from C-4 to C-5 of the electrophile and bearing the nucleophile at position 4 was obtained. Moreover, from the coupling between 1,3,5-tris(N-pyrrolidinyl)benzene and 4-chloro-7-nitrobenzofurazan a highly stable Wheland intermediate was isolated.

 Please wait while we load your content...
Please wait while we load your content...