Enantioselective organocatalytic oxidation of ketimine†
Abstract
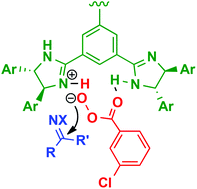
C 3-Symmetric chiral trisimidazolines with m-chloroperbenzoic acid promoted the organocatalytic oxidation of N-sulfonyl ketimine. The present imidazoline catalysis produced oxaziridines bearing a tetrasubstituted carbon stereogenic center in high yields with up to 87% ee.


 Please wait while we load your content...
Please wait while we load your content...