A versatile approach to flavones via a one-pot Pd(ii)-catalyzed dehydrogenation/oxidative boron-Heck coupling sequence of chromanones†
Abstract
A variety of flavones were expediently synthesized from readily accessible chromanones via a one-pot sequence involving Pd(II)-catalyzed dehydrogenation and oxidative boron-Heck coupling with arylboronic acid pinacol esters. In particular, the use of arylboronic acid pinacol esters was found to significantly improve the yield of the reaction.
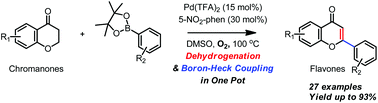

 Please wait while we load your content...
Please wait while we load your content...