Equation of state for water and its line of density maxima down to −120 MPa†
Abstract
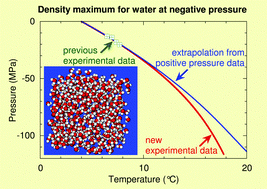
As water is involved in countless natural and industrial processes, its thermodynamic properties have been measured in a wide temperature and pressure range. Data on supercooled water are also available down to −73 °C and up to 400 MPa. In contrast, data at negative pressures are extremely scarce. Here we provide an experimental equation of state for water down to −120 MPa. In particular, we obtain the line of density maxima (LDM) of water down to a pressure six times more negative than previously available. As temperature increases from 4 up to 18 °C, the pressure PLDM(T) along the LDM decreases monotonically from 0 down to −120 MPa, while the slope dPLDM/dT becomes more negative. The experimental results are compared with molecular dynamic simulations of TIP4P/2005 water and a two-state model. We also discuss the possibility to observe extrema in compressibility and heat capacity at negative pressures, features that have remained elusive at positive pressures.

 Please wait while we load your content...
Please wait while we load your content...