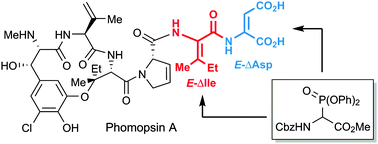
The stereoselective construction of E- and Z-Δ-Ile from E-dehydroamino acid ester: the synthesis of the phomopsin A tripeptide side chain†
Abstract
The stereoselective synthesis of the phomopsin A tripeptide side chain was achieved by using methyl 2-(((benzyloxy)carbonyl)amino)-2-(diphenoxyphosphoryl)acetate as a common synthetic precursor for the synthesis of E-Δ-dehydroisoleucine and E-Δ-aspartate.

 Please wait while we load your content...
Please wait while we load your content...