Substrate structure–activity relationships guide rational engineering of modular polyketide synthase ketoreductases†
Abstract
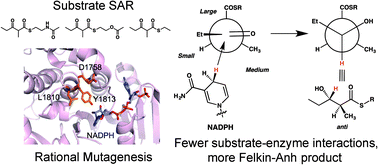
Modular polyketide synthase ketoreductases can set two chiral centers through a single reduction. To probe the basis of stereocontrol, a structure–activity relationship study was performed with three α-methyl, β-ketothioester substrates and four ketoreductases. Since interactions with the β-ketoacyl moiety were found to be most critical, residues implicated in contacting this moiety were mutated. Two mutations were sufficient to completely reverse the stereoselectivity of the model ketoreductase EryKR1, converting it from an enzyme that generates (2S,3R)-products into one that yields (2S,3S)-products.

 Please wait while we load your content...
Please wait while we load your content...