Chiral N-heterocyclic carbene/Lewis acid cooperative catalysis of the reaction of 2-aroylvinylcinnamaldehydes: a switch of the reaction pathway by Lewis acid activation†
Abstract
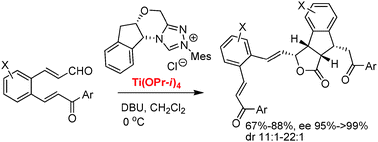
The chiral N-heterocyclic carbene/Lewis acid co-catalyzed reaction of 2-aroylvinylcinnamaldehydes produced good yields of indeno[1,2-c]furan-1-one derivatives with excellent enantioselectivity and diastereoselectivity. A switch of intramolecular cyclization to intermolecular dimerization was achieved by the cooperative catalysis of chiral triazole carbene and Ti(OPr-i)4 catalysts.

 Please wait while we load your content...
Please wait while we load your content...