A dinuclear gold(i) complex as a novel photoredox catalyst for light-induced atom transfer radical polymerization†
Abstract
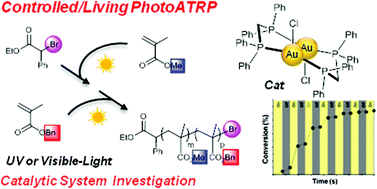
Controlled/living atom transfer radical polymerization of methacrylates and acrylates initiated by ethyl α-bromophenylacetate (EBPA) as the initiator in the presence of low loadings (1.25 mol% vs. initiator) of a dinuclear gold(I) complex based photocatalyst [Au2(μ-dppm)2]Cl2 has been accomplished in solution and in laminate under UVA and visible-light photoreductive conditions. In solution, the linear increase of molecular weights with methyl methacrylate (MMA) conversion and the low dispersity are consistent with a controlled/living process. In a film, trimethylolpropane triacrylate (TMPTA) was polymerized and the ethyl α-bromophenylacetate (EBPA)/[Au2(μ-dppm)2]Cl2 system resulted in a faster rate of polymerization compared to EBPA/Ir(ppy)3. Chain extensions of polymers were successfully conducted leading to block copolymers, which also confirms the living character of this new system. Photophysical experiments support a conventional photoredox-catalyzed ATRP mechanism. Finally, this approach utilizes a gold catalyst featuring better solubility and lower cost than the well-known Ir(ppy)3 complex.

 Please wait while we load your content...
Please wait while we load your content...