Highly water-soluble and tumor-targeted photosensitizers for photodynamic therapy†
Abstract
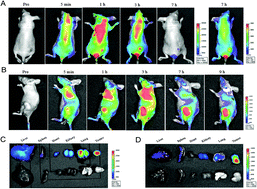
Biological uses of photosensitizers in photodynamic therapy (PDT) often suffer from a lack of tumor selectivity; a strategy based on molecule-targeted cancer therapies could provide a promising solution. To synthesize new water-soluble phthalocyanines (Pcs) for bio-conjugation with peptides or antibodies, we developed a method to synthesize asymmetrically substituted Pcs with both high water solubility and one monoamino group for conjugation with biological agents for tumor homing, using folic acid as the ligand model to direct the modified Pcs into target cells. Here, we report studies on the syntheses and characterization of these Pcs. In vitro and in vivo assays prove that the high solubility characteristic can greatly increase the tumor targeting capability of Pcs by reducing non-specific uptake. This newly designed photosensitizer accumulated almost completely in tumor regions, with a negligible signal found in other tissues in the xenograft tumor model. These initial data provide strong evidence of the high specificity tumor targeting of Pcs with folate and tri-glycerol substitutions. Theoretically, the synthesized Pcs could be conveniently conjugated to many other ligands, endorsing the broad applicability of this method for tumor-targeted PDT.

 Please wait while we load your content...
Please wait while we load your content...