Substrate geometry controls the cyclization cascade in multiproduct terpene synthases from Zea mays†
Abstract
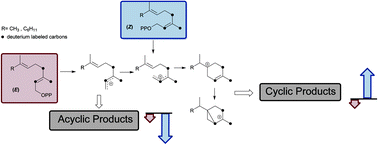
Multiproduct terpene synthases TPS4-B73 and TPS5-Delprim from maize (Zea mays) catalyze the conversion of farnesyl diphosphate (FDP) and geranyl diphosphate (GDP) into a complex mixture of sesquiterpenes and monoterpenes, respectively. Various isotopic and geometric isomers of natural substrates like (2Z)-[2-2H]- and [2,4,4,9,9,9-2H6]-(GDP) and (2Z,6E)-[2-2H]- and [2,4,4,13,13,13-2H6]-(FDP) were synthesized analogous to presumptive reaction intermediates. On incubation with labeled (2Z) substrates, TPS4 and TPS5 showed much lower kinetic isotope effects than the labeled (2E) substrates. Interestingly, the products arising from the deuterated (2Z)-precursors revealed a distinct preference for cyclic products and exhibited an enhanced turnover on comparison with natural (2E)-substrates. This increase in the efficiency due to (2Z) configuration emphasizes the rate limiting effect of the initial (2E) → (2Z) isomerization step in the reaction cascade of the multiproduct terpene synthases. Apart from turnover advantages, these results suggest that substrate geometry can be used as a tool to optimize the biosynthetic reaction cascade towards valuable cyclic terpenoids.

 Please wait while we load your content...
Please wait while we load your content...