Thiocyanation of BODIPY dyes and their conversion to thioalkylated derivatives†
Abstract
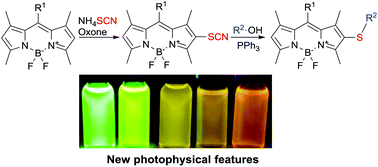
A high-yielding method for the direct thiocyanation of BODIPY dyes is described. In 1,3-dimethyl BODIPYs, the thiocyanato group adds at position 2, whereas the insertion occurs at position 5 in 3-amino BODIPYs. The transformation of the thiocyanato group enables the synthesis of thioalkylated BODIPYs. 2-Thioalkylated BODIPYs and 3-thiocyanato-5-piperidino BODIPYs exhibit interesting spectroscopical features. Hence, the described synthetic methodology can be used for the photophysical tuning of BODIPY dyes.


 Please wait while we load your content...
Please wait while we load your content...