Metabolic alkene labeling and in vitro detection of histone acylation via the aqueous oxidative Heck reaction†
Abstract
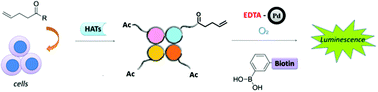
The detection of protein lysine acylations remains a challenge due to lack of specific antibodies for acylations with various chain lengths. This problem can be addressed by metabolic labeling techniques using carboxylates with reactive functionalities. Subsequent chemoselective reactions with a complementary moiety connected to a detection tag enable the visualization and quantification of the protein lysine acylome. In this study, we present EDTA-Pd(II) as a novel catalyst for the oxidative Heck reaction on protein-bound alkenes, which allows employment of fully aqueous reaction conditions. We used this reaction to monitor histone lysine acylation in vitro after metabolic incorporation of olefinic carboxylates as chemical reporters.

 Please wait while we load your content...
Please wait while we load your content...