Regioselective synthesis, antimicrobial evaluation and theoretical studies of 2-styryl quinolines†
Abstract
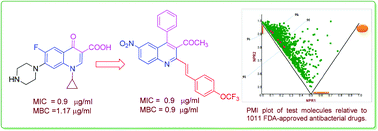
2-Styryl quinolines (9a–l) have been synthesized regioselectively from 2-methyl-quinoline by using NaOAc in water acetic acid binary solvents and evaluated for their antibacterial activity against both Gram-positive and Gram-negative strains. Among these, the compounds 12 and 8 were found to be active against both bacterial strains. Compounds 9b, 9f, 9g, 9i, 9j and 9k were the most active among the series exhibiting MIC values ranging between 1.9 and 31.2 μg ml−1 against different bacterial strains. Compounds 9j and 9k were found to be as potent as the standard drug ciprofloxacin against Micrococcus luteus, Klebsiella planticola and Staphylococcus aureus. In addition, the compounds showed bactericidal activity; compound 9j was found to be better than ciprofloxacin, with an MBC value of 0.9 μg ml−1 against both M. luteus and K. planticola. The compounds also inhibited biofilm formation, and compound 9j was found to be equipotent to erythromycin against M. luteus and S. aureus MLS16. Further, theoretical studies such as those on druggable properties and PMI plot have been carried out.

 Please wait while we load your content...
Please wait while we load your content...