A case of oxoanion recognition based on combined cationic and neutral C–H hydrogen bond interactions†
Abstract
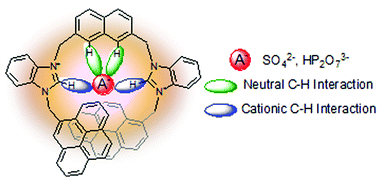
A novel bidentate bis-(benzimidazolium) receptor containing pyrene as fluorescent signaling units has been synthesized. Fluorescence and NMR spectroscopy studies reveal that this receptor exclusively recognizes sulphate and hydrogenpyrophosphate in the competitive water–DMSO (1 : 9) medium; significant downfield shifts were observed for the C(2)–H protons of both the imidazolium groups, and appreciable downfield shifts were also observed for the inner naphthalene protons indicating their participation in hydrogen bonding with anions along with the C(2) imidazolium protons. The calculated association constants from 1H NMR and fluorescence titrations demonstrate that the receptor binds sulphate stronger than hydrogenpyrophosphate anions.

 Please wait while we load your content...
Please wait while we load your content...