Gd(OTf)3-catalyzed synthesis of geranyl esters for the intramolecular radical cyclization of their epoxides mediated by titanocene(iii)†
Abstract
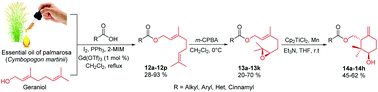
A selective and mild method for the esterification of a variety of carboxylic acids with geraniol is developed. We demonstrated that the use of triphenylphosphine, I2, 2-methylimidazole or imidazole and a catalytic amount of Gd(OTf)3 resulted to be more active than the previous protocols, providing a 16-membered library of geranyl esters in higher yields and in shorter reaction times. The use of essential oil of palmarosa (Cymbopogon martinii), enriched with geraniol, as a raw material for the synthesis of the target compounds complemented and proved how sustainable and eco-friendly this protocol is. Finally, the selective 6,7-epoxidation of the obtained geranyl esters led us to study their regio-controlled radical cyclization mediated by titanocene(III) for the synthesis of novel (8-hydroxy-9,9-dimethyl-5-methylene cyclohexyl)methyl esters in moderate yields and with excellent stereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...