Heteroleptic cationic iridium(iii) complexes bearing naphthalimidyl substituents: synthesis, photophysics and reverse saturable absorption†
Abstract
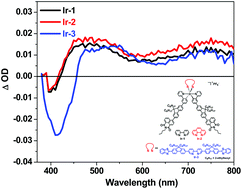
Three heteroleptic cationic iridium(III) complexes containing a cyclometalating 2-[3-(7-naphthalimidylfluoren-2′-yl)phenyl]pyridine ligand and different diimine (N^N) ligands (N^N = 2,2′-bipyridine (bpy, Ir-1), 1,10-phenanthroline (phen, Ir-2), and 5,5′-bis[7-(benzothiazol-2′-yl)fluoren-2′-yl]-2,2′-bipyridine (BTF-bpy, Ir-3)) were synthesized and characterized. The photophysics of these complexes was systematically investigated via spectroscopic methods and by time-dependent density functional theory (TDDFT). All complexes possess a very weak charge-transfer tail at ca. 450–570 nm; and two intense absorption bands in the region of 290–350 nm and 350–450 nm, respectively. The emission of Ir-1–Ir-3 in CH2Cl2 emanates predominantly from the C^N ligand-localized 3π,π* state. These emitting excited states also give rise to broadband triplet excited-state absorption in the visible to the near-IR region (i.e. 420–800 nm for Ir-1 and Ir-2, and 460–800 nm for Ir-3). The kinetics of fs transient absorption (TA) reveals that the lowest singlet excited-state lifetimes of these complexes vary from 1.43 ps to 142 ps. The stronger excited-state absorption of Ir-1–Ir-3 compared to their respective ground-state absorption in the visible spectral range leads to strong reverse saturable absorption (RSA) at 532 nm for ns laser pulses. The trend of transmission signal decrease follows Ir-2 > Ir-3 > Ir-1. Extending the π-conjugation of the N^N ligand increases the strength of RSA. In addition, the naphthalimidyl (NI) substitution at the cyclometalating ligand dramatically increases the triplet excited-state lifetimes and broadens the triplet excited-state absorption to the NIR region compared to the respective Ir(III) complexes with a benzothiazolyl substituent on the cyclometalating ligand.

 Please wait while we load your content...
Please wait while we load your content...