Non-symmetrical aryl- and arylethynyl-substituted thioalkyl-porphyrazines for optoelectronic materials: synthesis, properties, and computational studies†
Abstract
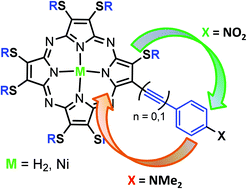
A series of novel non-symmetrically substituted mono β-aryl and β-arylethynyl (alkylsulfanyl)porphyrazines and the corresponding Ni(II) complexes have been prepared by the Suzuki–Miyaura and Sonogashira cross-coupling reactions with the aim to investigate substituent effects on their electronic and aggregation properties. Spectroscopic, electrochemical and computational investigations show that in both aryl and arylethynyl compounds efficient electron transfer between the aryl and macrocycle moieties occurs. The highest perturbation of the porphyrazine π-electron core is provided by strong electron-donating (NMe2) and electron withdrawing (NO2) aryl substituents, which increase and decrease the macrocycle electron density, respectively. Moreover, while in most of the compounds the LUMOs and HOMOs are mainly localized on the porphyrazine ring, in the amino-substituted derivatives the HOMO is localized on the peripheral aryl moieties and the LUMO is localized on the macrocycle. Charge-transfer electronic excitations give rise to absorptions in UV-Vis spectra of both amino- and nitro-substituted compounds. In the former such excitations occur from aryl-localized to macrocycle-localized orbitals, while backward excitations occur in the latter. Therefore, the porphyrazine ring shows an ambivalent behavior, acting as an electron acceptor in the case of the NMe2-substituted compounds and as an electron donor in the NO2-substituted derivative. In these derivatives, even macrocycle mono-substitution provides unconventional “push–pull” systems suitable for NLO. Columnar discotic mesophases are also shown by thio-octyl arylethynyl derivatives, allowing us to envisage the possibility to achieve compounds both suitable for optoelectronic applications and endowed with self-aggregation properties.

 Please wait while we load your content...
Please wait while we load your content...