Synthesis of BiVO4 nanoflake array films for photoelectrochemical water oxidation†
Abstract
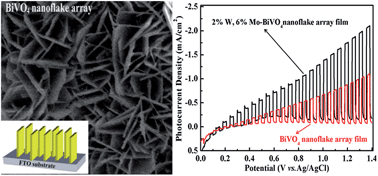
Because of the potential for application in photoelectrochemical cells for water splitting, the synthesis of nanostructured BiVO4 is receiving increasing attention. Here we report a simple new drop-casting method for the first time to synthesize un-doped and doped bismuth vanadate (BiVO4) nanoflake array films. Synthesis parameters such as the amount of polyethylene glycol 600 (PEG-600) and the precursor solution drying time are investigated to optimize the films for photoelectrochemical water oxidation. The BiVO4 films consisting of nanoflakes with an average thickness of 20 nm and length of 2 μm were synthesized from a precursor solution containing Bi3+, V3+ and PEG-600 with a Bi : V: PEG-600 volume ratio of 2 : 2 : 1, dried at 135 °C for 55 min. Photoelectrochemical measurements show that the BiVO4 nanoflake array films have higher photoelectrochemical activity than the BiVO4 nanoparticle films. Additionally, the nanoflake arrays were tested after incorporating W and Mo to enhance the photoelectrochemical activity. The 2% W, 6% Mo co-doped BiVO4 nanoflake array films demonstrate the best photoelectrochemical activity with photocurrent densities about 2 times higher than the un-doped BiVO4 nanoflake films and greater than the photocurrents of individually Mo doped or W doped BiVO4 films. The origin of enhanced photoelectrochemical activity for the co-doped film may be due to the improved conductivity through the BiVO4 or slightly enhanced water oxidation kinetics.

 Please wait while we load your content...
Please wait while we load your content...