The magnetism of Fe4N/oxides (MgO, BaTiO3, BiFeO3) interfaces from first-principles calculations
Abstract
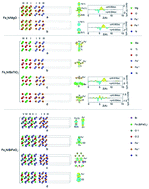
The geometry, bonding, electronic and magnetic properties of Fe4N/oxides (MgO, BaTiO3 and BiFeO3) interfaces with different configurations are performed using first-principles calculations. The n- and p-type doping of MgO are induced in FeIFeII/MgO and (FeII)2N/MgO interfaces, respectively. The metallic characteristics are induced in BaTiO3 by contact with FeIFeII termination, followed by p-type doping in the (FeII)2N/BaO interface and n-type doping in the (FeII)2N/TiO2 interface. The interfacial dipole due to charge rearrangement may induce the Fermi level pinning in the Fe4N/MgO and (FeII)2N/BaTiO3 systems. The deposition of Fe4N on BiFeO3 leads to a metallic character of BiFeO3 with total magnetic moments of 0.33–1.54 μB. The different electronic and magnetic characters are governed by interfacial bonding between Fe4N and oxides. These findings are useful for the future design of Fe4N/oxides based spintronics devices.

 Please wait while we load your content...
Please wait while we load your content...