Photochemical and Ga2O3-photoassisted decomposition of the insecticide Fipronil in aqueous media upon UVC radiation†
Abstract
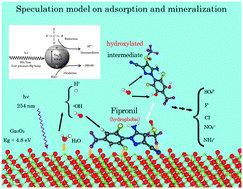
Fipronil is a phenylpyrazole-based insecticide and one of the relatively new widely used generations of insecticides whose biochemical action differs from the more traditional insecticides such as the organo-phosphates and -carbamates. Although several studies have been reported to determine the fate of Fipronil under environmental field conditions, including irradiation with UVB/UVA natural or simulated sunlight (wavelength >290–300 nm), which resulted in the formation of products that retained the basic phenylpyrazole structure, the present article examines the photodegradation of Fipronil upon irradiation of aqueous solutions under both reducing (nitrogen) and oxidative (air oxygen) atmospheric conditions at 254 nm (UVC; low-pressure Hg lamp), and for comparison purposes in the presence of the wide band-gap (4.8–5.0 eV) gallium sesquioxide (β-Ga2O3) semiconductor (and for comparison also TiO2). The fate of the insecticide under such conditions was ascertained by absorption spectroscopy in the UVC spectral region, by HPLC techniques for the desulfonation, defluorination, dechlorination and formation of both nitrate and ammonium ions, in addition to ESI-TOF-MS mass spectral methods to identify some of the possible intermediates that may have formed following the breakup of the phenyl and pyrazole rings. Mass spectra indicated that within 30 min filoprin was converted to Fipronil-desulfinyl (loss of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O fragment; photoproduct (III)) and in less than 1 hour of irradiation a significant quantity of the insecticide's photoproduct (III) had also decomposed as no significant mass peaks were seen above m/z = 319. The largest yields of SO42− (79%), F− (44%), Cl− (33%), NO3− (34%) and NH4+ (15%) were obtained under air-equilibrated conditions in the presence of the metal oxide β-Ga2O3 (Ga2O3/O2), followed by air O2, Ga2O3/N2 and under N2 atmospheric conditions. Frontier electron densities and partial charges on all the atoms of Fipronil were calculated (hf/6-31g* configuration; Gaussian 09 software) to infer a possible, albeit not detailed, pathway(s) for the direct photolysis and when the metal oxides were involved in the photodegradation. In addition, Ames tests were carried out on the intermediate products of the photodegradation of Fipronil after 3 h and 24 h of UVC illumination; none of the intermediates displayed mutagenic activity.
O fragment; photoproduct (III)) and in less than 1 hour of irradiation a significant quantity of the insecticide's photoproduct (III) had also decomposed as no significant mass peaks were seen above m/z = 319. The largest yields of SO42− (79%), F− (44%), Cl− (33%), NO3− (34%) and NH4+ (15%) were obtained under air-equilibrated conditions in the presence of the metal oxide β-Ga2O3 (Ga2O3/O2), followed by air O2, Ga2O3/N2 and under N2 atmospheric conditions. Frontier electron densities and partial charges on all the atoms of Fipronil were calculated (hf/6-31g* configuration; Gaussian 09 software) to infer a possible, albeit not detailed, pathway(s) for the direct photolysis and when the metal oxides were involved in the photodegradation. In addition, Ames tests were carried out on the intermediate products of the photodegradation of Fipronil after 3 h and 24 h of UVC illumination; none of the intermediates displayed mutagenic activity.

 Please wait while we load your content...
Please wait while we load your content...