Immobilization of polyoxometalate-based ionic liquid on carboxymethyl cellulose for epoxidation of olefins†
Abstract
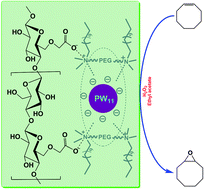
A novel ionic liquid consisting of a PEG-functionalized ammonium cation and a lacunary-type phosphotungstate anion was synthesized and characterized structurally. The ionic liquid was then immobilized onto environmentally benign polymer-carboxymethyl cellulose by two different routes: the impregnation method and the co-precipitation method. The immobilized ionic liquid can be used as a catalyst for olefin epoxidation with aqueous hydrogen peroxide in ethyl acetate. It was found that both of the immobilized ionic liquid catalysts showed better catalytic activities and stability than the homogeneous analogue in consecutive runs. In particular, the ionic liquid catalyst immobilized by the co-precipitation method afforded a higher catalytic stability than the catalyst immobilized by the impregnation method. It is suggested that the crucial factor influencing the catalytic performance is the difference in the interactions between the heteropoly anions and the polymer supports.

 Please wait while we load your content...
Please wait while we load your content...