Characterization of the extracted complexes of trivalent lanthanides with purified cyanex 301 in comparison with trivalent actinide complexes
Abstract
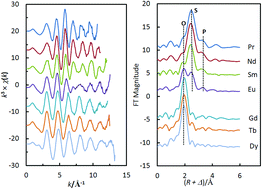
The extracted complexes of trivalent lanthanides (LnIII) with purified Cyanex 301 (bis(2,4,4-trimethylpentyl)dithiophosphinic acid, denoted as HA) were investigated by extended X-ray absorption fine structure spectroscopy (EXAFS), UV-Vis and fluorescence spectroscopy. In the complexes prepared under the same conditions of solvent extraction, the light LnIII ions are mainly coordinated by the sulfur atoms of the ligands, and the middle LnIII ions are coordinated by mixed donors, the sulfur atoms of the ligands and the oxygen atoms of the extracted water, while the heavy LnIII ions are completely hydrated in the organic phase without any sulfur atoms of the ligands in the coordination shell. As the atomic number increases, the extracted water molecules gradually replace the sulfur atoms of the ligands in the first coordination shell of LnIII, and simultaneously the ligand anions become counterions just for balancing the positive charge of the fully hydrated heavy LnIII ions. The effect of the change in the complex structures on the extraction of LnIII ions with HA was evaluated by the co-extraction of other thirteen individual LnIII together with NdIII. In contrast to most ligands bonding more strongly to heavier LnIII, HA preferentially extracts lighter LnIII, suggesting that the unusual extraction capability of HA for LnIII might originate from the difference in the complex structures with LnIII ions.

 Please wait while we load your content...
Please wait while we load your content...