Heat and mass transfer through interfaces of nanosized bubbles/droplets: the influence of interface curvature
Abstract
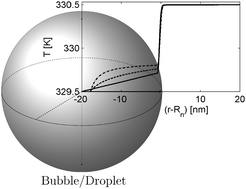
Heat and mass transfer through interfaces is central in nucleation theory, nanotechnology and many other fields of research. Heat transfer in nanoparticle suspensions and nanoporous materials displays significant and opposite correlations with particle and pore size. We investigate these effects further for transfer of heat and mass across interfaces of bubbles and droplets with radii down to 2 nm. We use square gradient theory at and beyond equilibrium to calculate interfacial resistances in single-component and two-component systems. Interface resistances, as defined by non-equilibrium thermodynamics, vary continuously with the interface curvature, from negative (bubbles) to zero (planar interface) to positive (droplet) values. The interface resistances of 2 nm radii bubbles/droplets are in some cases one order of magnitude different from those of the planar interface. The square gradient model predicts that the thermal interface resistances of droplets decrease with particle size, in accordance with results from the literature, only if the peak in the local resistivity is shifted toward the vapor phase. The curvature will then have an opposite effect on the resistance of bubbles and droplets. The model predicts that the coupling between heat and mass fluxes, when quantified as the heat of transfer of the interface, is of the same order of magnitude as the enthalpy change across the interface, and depends much less on curvature than the interface resistances.

 Please wait while we load your content...
Please wait while we load your content...