Effect of fluorine substitution on structures and reactivity of Keggin-Al13 in aqueous solution: an exploration of the fluorine substitution mechanism†
Abstract
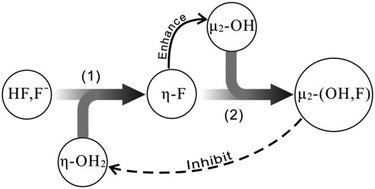
The structures and reactivity of fluoridated Keggin-aluminum tridecamers (K-Al13) in aqueous solution were studied using density functional theory (DFT) in order to explore the fluorine substitution process and the influence of F− on the chemical behavior of Keggin polynuclear Al species. The structures of fluoridated K-Al13 were optimized with the consideration of both explicit and bulk solvent effects. The obtained optimization parameters indicate that the fluorine substitution on distinct sites results in the different structural changes. The consistency between the computational and experimental 19F and 27Al NMR chemical shifts validates the suitable computational method for the present clusters. According to the computed energy barriers of water exchange reactions, it was found that the reactivity of η-OH2 decreases as a function of fluorine bridges. The appearance of fluorine bridges does not change the dissociative activated mechanism, but the larger dissociative trend was observed. Combining the present computational results with previous experimental observations, we reasonably put forward the fluorine substitution process and attempt to interpret the experimental phenomenon concerned at the molecular scale and further reveal the mechanism of F− promoting dissolution of mineral.

 Please wait while we load your content...
Please wait while we load your content...