Hydration properties of Cm(iii) and Th(iv) combining coordination free energy profiles with electronic structure analysis†
Abstract
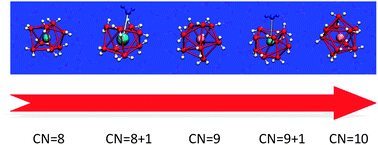
The hydration structure of two actinoid ions of different charge, Cm(III) and Th(IV), was investigated. Density Functional Theory, DFT-based molecular dynamics and the single sweep method were used to obtain free energy landscapes of ion–water coordination. Free energy curves as a function of the ion–water coordination number were obtained for both ions. The number of water molecules in the first coordination shell of Cm(III) varies between 8 and 10. For Th(IV), on the other hand, the 9-fold structure is stable and only the 10-fold structure seems to be accessible with a small but non-negligible free energy barrier. Finally, by combining molecular dynamics simulations with electronic structure calculations, we showed that the differences between Cm(III) and Th(IV) are mainly due to electrostatic effects. Cm(III) is less charged and it has fewer water molecules in its first shell, while Th(IV) has more water molecules because of a stronger electrostatic interaction.

 Please wait while we load your content...
Please wait while we load your content...