Ruthenium-modified zinc oxide, a highly active vis-photocatalyst: the nature and reactivity of photoactive centres
Abstract
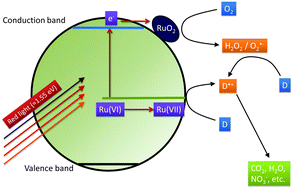
We recently reported a highly active photocatalyst, ruthenium-modified zinc oxide, which was found to be able to utilise the red part of the visible light spectrum for photocatalytic reactions [Bloh et al., Environ. Sci. Pollut. Res., 2012, 19, 3688–3695]. However, the origin and mechanism of the observed activity as well as the nature of the photoactive centres are still unknown. Herein, we expand on that by reporting a series of experiments specifically designed to unravel the mechanism of the visible light induced photocatalytic reactions. The absolute potentials of the valence and the conduction band edge are identified by the combined use of electrochemical impedance and UV-vis diffuse reflectance spectroscopy. The conduction band electron and the valence band hole activity are assessed through a novel approach tracing their signature oxidative species, i.e., hydrogen peroxide and hydroxyl radicals, respectively. Oxygen reduction currents are measured at different potentials to investigate the role of molecular oxygen as an electron scavenger as well as the underlying reduction pathways. Additionally, the photocatalytic activity of the samples is verified using another (ISO standard) degradation test, the gas-phase oxidation of nitric oxide. The experimental results reveal that the employed synthetic route yields a unique mixture of ruthenium(VI)-doped zinc oxide and ruthenium(VI) oxide particles with both forms of the ruthenium playing their own independent role in the enhancement of the photocatalytic activity. The ruthenium ions acting as dopants enable a better charge separation as well as the absorption of red light resulting in the direct promotion of electrons from the Ru(VI)-species to the conduction band. Both, the conduction band electrons and the thus formed Ru(VII) subsequently participate in the degradation of the pollutant molecules. The ruthenium dioxide particles, on the other hand, act as catalysts increasing the efficiency of the reaction by improving the oxygen reduction properties of the material.

 Please wait while we load your content...
Please wait while we load your content...