Multinuclear coordination polymers based on Ag⋯Ag interaction: syntheses, structures, and luminescence properties†
Abstract
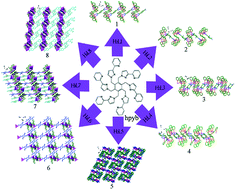
A series of coordination polymers based on Ag⋯Ag interactions, namely, [Ag2(hpyb)0.5(L1)0.5(NO3)]·H2O (1), [Ag4(hpyb)(HL2)(NO3)2]·2H2O (2), [Ag3(hpyb)0.5(L3)(NO3)] (3), [Ag6(hpyb)(L4)2(NO3)]·NO3·2H2O (4), [Ag5(hpyb)0.5(L5)2(NO3)]·H2O (5), {Ag4(hpyb)[L6(CH3)2]2} (6), {Ag6(hpyb)(HL7)2[L7(CH3)]} (7) and [Ag3(hpyb)0.5(HL8)]·H2O (8) (H2L1 = p-phthalic acid, H3L2 = 1,2,3-benzenetricarboxylic acid, H2L3 = cis-2-butenedioic acid, H2L4 = 2,3-pyridinedicarboxylic acid, H2L5 = m-phthalic acid, H4L6 = 1,2,4,5-benzenetetracarboxylic acid, H3L7 = 1,2,4-benzenetricarboxylic acid and H4L8 = 4,4′-oxydiphthalic acid) has been synthesized. For compounds 1–4, the polycarboxylate anions bridge multinuclear Ag(I) units to form 1D chains, respectively. The chains are extended by π–π interactions into a 2D supramolecular layer for compound 3 and 3D supramolecular architectures for compounds 1, 2 and 4. Compound 5 displays a 3D (4,8)-connected (34·42)(34·412·58·64)2 framework. Compounds 6–8 exhibit 2D layers, where the layers of 6 and 8 are further linked by π–π interactions to yield 3D supramolecular architectures. In the solid state, compounds 1–8 exhibit strong fluorescence emission bands at room temperature.

 Please wait while we load your content...
Please wait while we load your content...